Solutions for Global Bisphenol Challenges
GG Organics Pvt. Ltd., has been providing technology, development and technical services to the leather industry for over decades and strives hard to put the customer and the environment foremost.
GG Organics is the first Indian company to provide solutions to the global leather industry for reducing the Bisphenol A, F, S, and AF content of synthetic retanning agents. BPA is not present in any of their retanning agents.
GG Organicshas developed phenol-based retanning agents with desired limits of bisphenol content by using an innovative process, technology and monomer chemistry. The leather treated with GG Organics' low bisphenol retanning syntans has physical and mechanical properties that are similar to regular phenol and sulphone syntans, resulting in high-quality leather.
GG Organics’ cutting-edge laboratory has been upgraded with advanced LC-MS testing equipment for identifying the concentration of bisphenol in retanning agents and leather. This would ensure an efficient delivery mechanism in the frontier areas of the leather industry.
Bisphenol Restrictions:
- BPA has been restricted as a substance on its own and in mixtures intended for consumer use in the EU since March 2018.
- The Use of BPA in thermal paper has been restricted since January 2020.
- Germany has submitted an intention in 2020 to limit Bisphenols of similar concern (BOsC) in the future with a proposed limit (200 ppm).
- In 2021, The “Call for Evidence of BAuA (Federal Institute for Occupational Safety andHealth)” for restriction of BPA and bisphenols of similar concern (BosC) proposed a new limit (10ppm) for leather and textile auxiliaries including articles (X20 lower than the current level).
- In April 2022, ECHA and the Member States assessed a group of 148 bisphenols and recommended that more than 30 bisphenols need to be restricted due to their potential hormonal or reprotoxic effects. (source: https://echa.europa.eu/de/-/group-assessment -of-bisphenols-identifies-need-forrestriction)
- The publication of the proposed restrictions has been delayed until October 2022
- Final enforcement of any restrictions is expected between 2024 and 2026.
Voluntary Restrictions by the brands
(Source:https://www.leatherworkinggroup.com/contentfiles/LWG-1161.pdf?v=1)
| Customers |
Bisphenol A |
Bisphenol F |
Bisphenol S |
| Kering |
≤200 ppm |
≤ 200 ppm |
≤ 1.000 ppm |
| Chanel |
|
Information |
Information |
| Salvatore
Ferragamo |
≤ 1.000 ppm (adults)
≤ 20 ppm (Children) |
≤ 1.000 ppm (adults)
≤ 20 ppm (children) |
≤ 1.000 ppm (adults)
≤ 20 ppm (children) |
| Hermes |
≤ 100 ppm |
≤ 100 ppm |
≤ 100 ppm |
| Prada |
≤ 1.000 ppm |
≤ 1.000 ppm |
≤ 1.000 ppm |
| Burberry |
≤ 1 on
polycarbonateplastics |
|
|
Why there is a demand for chemicals and leather with low bisphenol content?
Bisphenol and its derivatives are known endocrine descriptors that are harmful to both human health and the environment, as well as causing reproductive toxicity. Because of the adverse effects caused by Bisphenols, European Chemicals Agency (ECHA) and the EU Member States have started screening data on a large group of Bisphenols such as BPA, BPS,BPF, BPAF and their derivatives outset in 2020.
Regulations governing the use of Bisphenols are becoming more challenging for the leather industry, as the majority of synthetic retanning agents are phenol and sulphone based.
Though the limitation of bisphenol S in leather is expected to be implemented between 2024and 2026, manufacturers and brands have started asking for leather with less bisphenol without compromising product quality.
Due to that, there is an increasing demand for Bisphenol-free or low bisphenol leather and chemicals in the global leather market.
What is Bisphenol?
Bisphenol is a synthetic organic compound containing 2 phenol groups (two hydroxyl phenylgroups) and it is used mainly for making plastics and resins.
Types of Bisphenol
1. Bisphenol A (BPA)
2. Bisphenol AF (BPAF)
3. Bisphenol F (BPF)
4. Bisphenol S (BPS)
Bisphenol A (BPA)
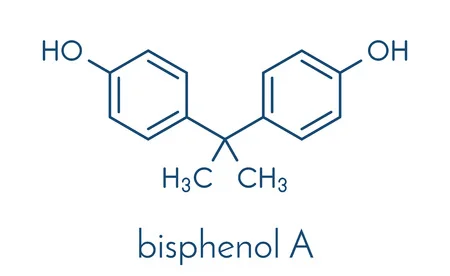
Chemical Formula: C15H16O2
IUPAC Name: 4,4'-(propane-2,2-diyl) diphenol
BPA is an organic chemical synthesised by the condensation of phenol and aceton.All by- products of the BPA synthesis, including unreacted phenol, are toxic. An environmental profile associated with the production of BPA as a monomer is available from plastic manufacturers. Lab-scale yields up to 96%
were reported for this process. A purity greater than 98% is required for most uses of BPA.
- It is widely used in the production of plastics, including polycarbonates and epoxy resins.
- 65-70% of all bisphenol A is used to make polycarbonate plastics.
- Polycarbonate plastic is used in products such as CDs, DVDs and dental materials. It can also be used in plastic bottles and lunch boxes.
- About 25-30% of all BPA is used in the manufacture of epoxy resins and vinyl ester resins
- Epoxy plastic can be used in electronics, building materials, in adhesives as an internalprotective coating in canned food products and soda bottles, and in the renovation ofwaste pipes.
- Bisphenol A is also present in thermal paper, which is used for receipts and tickets. Tetrabromobisphenol A (TBBPA) is a brominated flame retardant used in electronic equipment and thermoplastics.
- Some bisphenols are used as laboratory reagents.
Health Impacts
- BPA alters endocrine function, thereby causing many diseases in human and animals.
- Fatal development: miscarriages & low birth weight at term, according to studies.
- Altered immune system activity.
- Obesity and metabolic dysfunctions and diabetes in adults.
- Cardiovascular disease in adults.
- Cognitive and behavioural development in young children.
Regulations:
In June 2017, BPA was identified as having endocrine disrupting properties for human healthby the Member State Committee of the European Chemicals Agency (ECHA) (ECHA, 2017).
Due to its properties as toxic for reproduction, BPA was already listed as a substance of veryhigh concern (SVHC) on the Candidate List under Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH).
Hazard Classification and Labelling
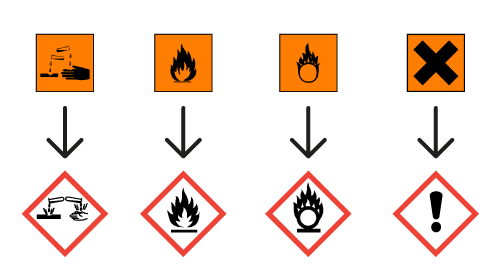
Danger! According to the harmonised classification and labelling (ATP09) approved by the European Union, this substance may damage fertility, causes serious eye damage, may causean allergic skin reaction and may cause respiratory irritation.
Additionally, the classification provided by companies to ECHA in REACH registrations identifies that this substance may damage fertility or the unborn child and is toxic to aquatic life with long-lasting effects.
Bisphenol AF (BPAF)
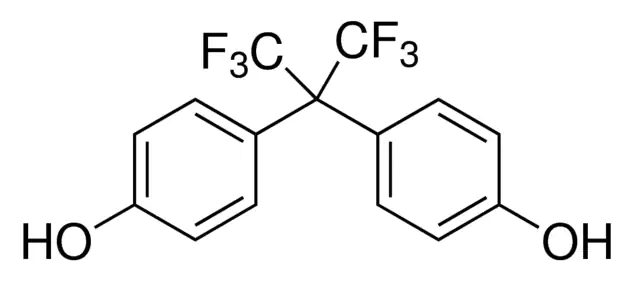
Chemical Formula: C15H10F6O2
IUPAC Name: 4,4′-(1,1,1,3,3,3-Hexafluoropropane-2,2- diyl)diphenol/
4,4'-[2,2,2-trifluoro-1- (trifluoromethyl)ethylidene]diphenol
BPAF is a fluorinated organic compound that is a derivative of bisphenol A in which thetwo methyl groups are replaced with trifluoromethyl groups.
- BPAF is used as polymer formulation and re-packing in the laboratory chemicals. Thissubstance may be released into the environment as a result of industrial use, such asformulation of mixtures and formulation in materials.
- BPAF is also used for the manufacture of rubber products.
Hazard Classification and Labelling
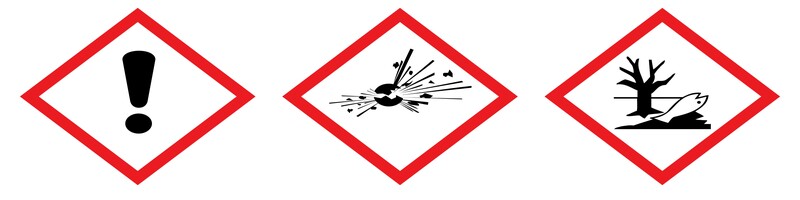
Danger! According to the classification provided by companies to ECHA in REACH registrations this substance may damage fertility or the unborn child, is very toxic to aquatic life with long lasting effects, causes serious eye damage and may cause damage to organs through prolonged or repeated exposure.
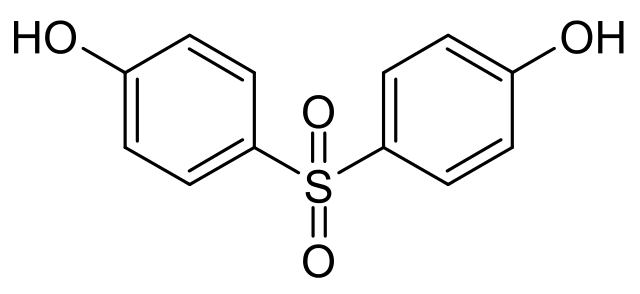
Chemical Formula: C12H10O4S
IUPAC Name: 4,4'-sulphonyldiphenol
BPS is an organic compound with two phenolic functional groups are on either side ofa sulfonyl group. BPS differentiates from BPA by possessing a sulfone group (SO2) as the central linker of the molecule instead of a dimethylmethylene group (C(CH3)2).
BPS is the reaction product of two moles of phenol with one mole of sulfuric acid. Along with4’-4’ sulphonyl bisphenol, 2-2,2,4 isomers are also formed.
2 C6H5OH + H2SO4 → (C6H4OH)2SO2 + 2 H2O
BPS or its derivatives are used in the production of polyethersulfone, polysulfones and epoxyresins
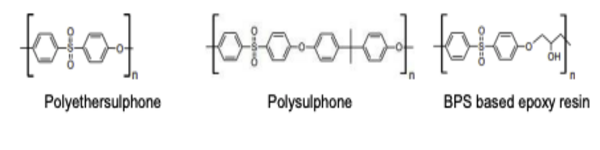
- BPS is mainly used in curing fast-drying epoxy resin adhesives.
- BPS is commonly used as monomer in the manufacturing of synthetic retanning agents
- It is used to make hard plastic and synthetic fibers
- It is commonly used as a replacement for BPA in some types of paper receipts, canbe used to lengthen color life in fabrics, and is a food packaging preservative.
- Bisphenol S can be found in: Thermal paper cash receipts, Plastics, Indoor dust, Medical devices and linings of beverage and food cans.
- BPS can be found in products with material based on: leather (e.g. gloves, shoes, purses, furniture), paper (e.g. tissues, feminine hygiene products, nappies, books, magazines, wallpaper), paper used for articles with intense direct dermal (skin) contact during normal use such as printed articles (e.g. newspapers, books, magazines, printed photographs).
- BPS is used in the production of sulphone syntans.
Health Impacts:
- BPS has endocrine disrupting properties similar to that of BPA. In a study of human urine, BPS was found in 81% of the samples tested.
- BPF, BPS, BPAF, along with Bisphenol Z (BPZ), Bisphenol E (BPE) and Bisphenol B (BPB)are suspected to be endocrine disrupting chemicals which are oestrogenic
- BPS has been shown to increase the expression of breast cancer carcinogens and theproliferation of positive breast cancer cells.
- BPS exposure has been linked to impaired neural function.
- High levels have been found to be significantly correlated with insulin resistance, albuminuria, and irregular vascular function in children.
- Pregnant women or women looking to become pregnant should reduce their exposure to Bisphenol S because it has been shown to reduce egg viability and may reduce fertility outcomes.
Hazard Classification and Labelling
Danger! According to the classification provided by companies to ECHA in REACHregistrations this substance may damage fertility or the unborn child.
Other release to the environment of this substance is likely to occur from: indoor use in long-life materials with low release rate (e.g. flooring, furniture, toys, construction materials, curtains, foot-wear, leather
products, paper and cardboard products, electronic equipment),outdoor use in long-life materials with low release rate (e.g. metal, wooden and plastic construction and building materials) and indoor use in long-life materials with high release rate (e.g. release from fabrics, textiles during washing, removal of indoor paints).
Bisphenol F
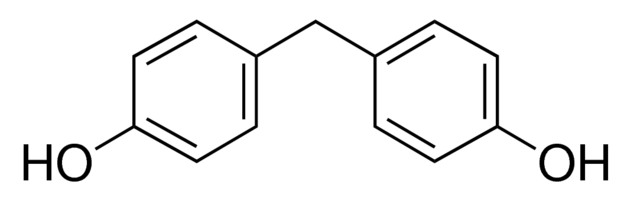
Chemical Formula: (HOC6H4)2CH2
IUPAC Name: 4,4′-dihydroxydiphenylmethane
BPF is an organic compound in which the two phenolic groups are linked bya
methylene connecting group. The Bisphenol F, is prepared by condensation reaction of phenol and formaldehyde with a large excess of phenol under acidic conditions. It is also formed during condensation of phenolic syntans reactions. It is structurally relatedto bisphenol A, a popular and controversial plasticizer, as both belong to the category of molecules known as bisphenols.
- BPF is used in the manufacture of plastics and epoxy resins.
- It is used in the production of tank and pipe linings, industrial flooring, road and bridgedeck toppings, structural adhesives, grouts, coatings and electrical varnishes.
- BPF is also utilized in liners, lacquers, adhesives, plastics, and the coating of drinks andfood cans.
- BPF is found in dental materials, such as restorative materials, liners, adhesives, oralprosthetic devices and tissue substitutes.
Hazard Classification and Labelling

Danger! According to the classification provided by companies to ECHA in REACH registrations this substance may damage fertility or the unborn child, is very toxic to aquatic life with long lasting effects, causes serious eye damage and may cause damage to organs through prolonged or repeated exposure.
Relationship between Bisphenol and leather
Replacement syntans are also known as synthetic organic tanning agents. This replacement syntans were initially developed to replace vegetable tannins, resulting in leathers with good softness and fullness.
Replacement syntans are classified into several types, but they are primarily composed of formaldehyde condensates of phenolsulphonic or naphtholsulphonic acids. In addition, cresol, napthol and bisphenol are also used for the manufacturing process of replacement syntans.
In particular, Bisphenol S (dihydroxy diphenyl sulphone) is used as raw materials for the manufacturing of sulphone syntan. During the course of reaction, bisphenol F may also formed when phenol reacts with formaldehyde. In the leather industry, these phenol/sulphone syntans are primarily used for pretanning, tanning, retanning, and dyeing.
Sulphone syntans has very good water dispensability, permeability and absorbability, especially with excellent light fastness and yellowing resistance. These syntans has been developed as a potential retanning agent for the production of wet-white leathers.
Bisphenol S is mainly used as a primary tanning agent in the manufacturing of light fast whitesyntans, and can also be used for dye fixing on leather and textiles.
Advantage of replacement syntans over vegetable tannins
- Good Light Fastness
- Yellowing resistance
- Softness
- No Iron stains
- Better physical properties
References:
1. https://www.hbm4eu.eu/hbm4eu-substances/bisphenols/
2. https://saferchemicals.org/get-the-facts/toxic-chemicals/bpa-bps/
3. https://sinlist.chemsec.org/chemical-groups/bisphenols/
4. https://www.millionmarker.com/the-science/chemical-glossary/bisphenol-s-bps
5. https://enveurope.springeropen.com/articles/10.1186/s12302-021-00586-9
6. https://journals.uc.edu/index.php/JALCA/article/view/1533/1039
7. Dihydroxy–diphenylsulphone and Salicylic Acid Derivatives in the Aftertreatment of Dyed Nylon M. Tomita,M. Tokitaka
8. Chemistry of Syntans and Their Influence on Leather Quality by Jochen Ammenn,* C. Huebsch, E. Schilling and B. Dannheim Automotive Leather, BASF SE, Ludwigshafen, Germany
9. https://www.chemicalbook.com/Article/Bisphenol-S-Chemical-properties-Uses.htm
10.https://www.reach-clp-biozid-helpdesk.de/EN/Home/German_propsal_restriction/BPA/BPA.html
For more information on the low bisphenol range of products, please contact
tech.support@ggorganics.co.in








